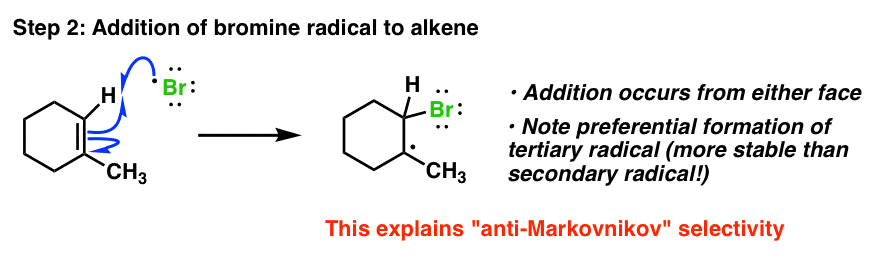
Robinson Annulation Mechanismbetween 2 Methylcyclohexene And Methyl Vinyl Ketone

The newly formed enolate intermediate must first tautomerize for the conversion to continue.
Robinson annulation mechanismbetween 2 methylcyclohexene and methyl vinyl ketone. 3 methyl 2 cyclohexen 1 one exists as a solid slightly soluble in water and an extremely weak acidic. Investigation in the field of stereochemistry of polycyclic compounds monoesters of cis and trans 1 methyl 1 2 cyclohexanedicarboxylic acids or 1 methyl 4 cyclohexene 1 2 dicarboxylic acids and their conversion. 2 methylcyclohexanone is a cyclic ketone that is reported to occur in mint and horse chestnut. 2 methylcyclohexanone exists as a liquid slightly soluble in water and an extremely weak basic essentially neutral compound based on its pka.
Mechanism of the robinson annulation. The reaction involves two important steps. Packaging 1 kg in glass bottle 100 g in glass bottle other notes download our flavors and fragrances catalog to view our entire product line. 2 methylcyclohexanone also known as fema 3946 or tetrahydro o cresol belongs to the class of organic compounds known as cyclic ketones.
Robinson annulation is a fundamental organic reaction in particular because it is at the base of the synthesis of steroids and other important molecules even of pharmaceutical interest. In a second step the methyl group adjacent to the carbonyl is deprotonated and undergoes an. The annulation reaction has as its most common substrates generally cycle hexanone and methyl vinyl ketone. 3 methyl 2 cyclohexen 1 one also known as mch or fema 3360 belongs to the class of organic compounds known as cyclohexenones.
The first step in the process is the michael addition to an α β unsaturated ketone such as methyl vinyl ketone ethyl vinyl ketone is shown above. Cyclohexenones are compounds containing a cylohexenone moiety which is a six membered aliphatic ring that carries a ketone and has one endocyclic double bond. As illustrated in the following figure the robinson annulation reaction involves two steps the first step involves the michael addition of a ketone enolate the enolate derived from cyclohexanone in the example pictured below to an alpha beta unsaturated ketone methyl vinyl ketone below. The optimized procedure 6 2 methyl 2 3 oxobutyl 1 3 cyclohexanedione 3 is prepared by heating 2 methylcyclohexane 1 3 dione 2 with methyl vinyl ketone 1 in aqueous acetic acid at 75 c for 1 h after which 3 is purified.
Pubmed the action of t butyl hypochlorite on 1 methyl 4 isopropyl 3 cyclohexene p menthene 3. The journal of organic chemistry 45 p. 1 michael addition 2 intramolecular aldol condensation.